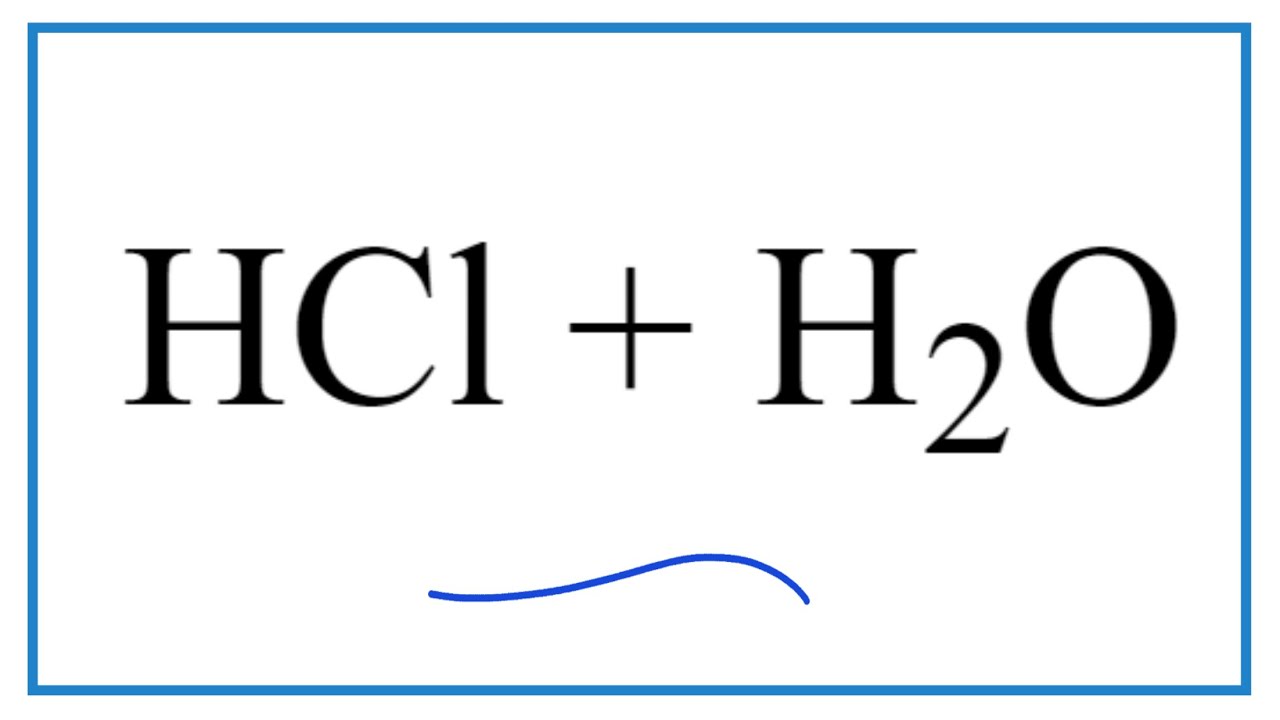Understanding Hydrochloric Acid Synthesis
Hydrochloric acid, a potent and widely used chemical, plays a crucial role in various industrial processes and laboratory applications. Understanding its properties, synthesis, and safe handling procedures is essential for anyone working with this compound. This article explores the various aspects of hydrochloric acid production, including its formula (HCl), historical context, and important safety considerations.
The journey of understanding hydrochloric acid begins with its fundamental chemical formula, HCl. This simple representation denotes one hydrogen atom bonded to one chlorine atom. However, the simplicity of the formula belies the complex reactions involved in its production. Creating hydrochloric acid requires careful control and understanding of chemical principles to ensure safety and efficiency.
Historically, hydrochloric acid was produced through various methods, including the heating of salt (sodium chloride) with sulfuric acid. This process released hydrogen chloride gas, which was then dissolved in water to form hydrochloric acid. Today, the primary industrial method involves the direct reaction of hydrogen gas and chlorine gas, a highly exothermic process yielding gaseous hydrogen chloride that is subsequently absorbed into water.
The significance of hydrochloric acid stems from its diverse applications. It's a crucial component in the production of various chemicals, including polyvinyl chloride (PVC), and is widely used in metal cleaning, processing, and refining. In the food industry, it's employed in the production of ingredients like gelatin and fructose. Moreover, hydrochloric acid plays a vital role in our own bodies, where it forms the primary component of gastric acid, aiding digestion in the stomach.
However, the production and handling of hydrochloric acid present several critical issues. Its corrosive nature poses significant risks, requiring stringent safety measures. Exposure to hydrochloric acid can lead to severe burns to skin, eyes, and respiratory tract. Furthermore, its production can generate environmentally harmful byproducts if not properly managed. Therefore, understanding the hydrochloric acid formula and its implications is only the first step; understanding safe and responsible handling is equally paramount.
The reaction between hydrogen and chlorine gas produces gaseous hydrogen chloride which is then dissolved in water to create hydrochloric acid. This reaction is highly exothermic, meaning it releases significant heat.
One benefit of understanding how to create hydrochloric acid is the ability to better control the reaction and ensure safety. Another benefit is the potential for optimizing the process for increased efficiency and yield. Lastly, understanding the underlying chemistry allows for better handling and disposal practices, minimizing environmental impact.
Advantages and Disadvantages of Producing Hydrochloric Acid
| Advantages | Disadvantages |
|---|---|
| Essential for various industrial processes | Highly corrosive and requires careful handling |
| Relatively inexpensive to produce | Production can generate harmful byproducts if not managed properly |
| Wide range of applications across different industries | Transportation and storage can be hazardous |
Creating hydrochloric acid involves a controlled chemical reaction and should only be attempted by trained professionals in equipped laboratories following strict safety protocols.
Frequently Asked Questions
1. What is the formula for hydrochloric acid? HCl
2. Is it safe to make hydrochloric acid at home? No, it is extremely dangerous and should only be done in controlled laboratory settings.
3. What are the main uses of hydrochloric acid? Industrial processes, metal cleaning, food production, and as a component of gastric acid.
4. What are the safety precautions when handling hydrochloric acid? Wear appropriate personal protective equipment (PPE) such as gloves, goggles, and respirators.
5. What are the environmental concerns related to hydrochloric acid production? Potential release of harmful byproducts if the process is not properly managed.
6. What happens if hydrochloric acid comes into contact with skin? It can cause severe burns and requires immediate medical attention.
7. Can hydrochloric acid be neutralized? Yes, with bases like sodium hydroxide.
8. What are some alternatives to hydrochloric acid for certain applications? Depending on the application, alternatives like citric acid or acetic acid may be suitable.
Tips and Tricks: Always work in a well-ventilated area. Double-check all equipment for leaks before starting the process.
In conclusion, understanding how hydrochloric acid is formed through its formula, HCl, and the reaction between hydrogen and chlorine is critical for appreciating its properties and safe handling. While the process appears straightforward on paper, the reality involves meticulous control and stringent safety measures due to the corrosive nature of hydrochloric acid. Its industrial importance is undeniable, contributing to various sectors from manufacturing to food processing. However, responsible production and handling are essential to minimize risks and environmental impact. Recognizing the significance of proper safety procedures, thorough training, and environmentally conscious practices are crucial for anyone working with this powerful chemical compound. The benefits of utilizing hydrochloric acid responsibly are numerous, but the potential hazards necessitate a cautious and informed approach. By understanding the chemical principles, adhering to safety protocols, and promoting responsible practices, we can harness the power of hydrochloric acid while mitigating its risks.
Boarding school boy to political firebrand unpacking tucker carlsons early years
The enduring legacy of ian mckellen a titan of stage and screen
The siege at bastogne a pivotal moment in wwii